Planck’s Law
When Max Planck decided on a career in physics, Munich professor Philipp von Jolly advised him against it on the basis that there was little left to discover in the field. Little did he know at the time that Planck would go on to earn a Nobel Prize in physics in 1918 for his discoveries and become one of the most admired scientists of all time.

One Sunday afternoon in 1900, Physicist Max Planck was entertaining fellow physicist Heinrich Rubens in his home in Berlin. Rubens was an accomplished experimentalist and was working on a problem called “The Ultraviolet Catastrophe” for several years, although it wasn’t called that until 1911. This was a case where experimental data was not lining up with theoretical, mathematical equations that physicists had devised up to that point. After Rubens left, Planck spent the rest of the evening trying come up with a more accurate, less catastrophic equation to fit Ruben’s experimental data.
Max Planck Institute of Physics
In one evening, Planck managed to derive a mathematical equation that was in excellent agreement with Ruben’s experimental data. Before retiring to bed that evening, he jotted down his now famous blackbody radiation formula onto a postcard and mailed it to Rubens. What Planck managed to do was reverse engineer the data Ruben’s had accumulated, and arrive at his results by “lucky guess-work.” He came up with a “fudge” number now known as Planck’s Constant “h” to make the math work. Planck’s constant = 6.62607004 × 10-34 m2 kg/s is now ubiquitous throughout the field of quantum physics. At that time, Planck did not have a physical interpretation for his formula, he just knew that the numbers worked.
Planck worked for two months to come up with what is now knows as “Planck’s Law,” which describes energy as manifesting in the form of discrete levels, now called “quanta,” instead of the linear manifestation that nearly everyone assumed up to that point. He presented his findings to the German Physical Society on December 14, 1900, the day now widely regarded as the birthday of quantum physics and also a demarcation point between the eras of “classical physics” and “quantum physics.”
One Sunday afternoon in 1900, Physicist Max Planck was entertaining fellow physicist Heinrich Rubens in his home in Berlin. Rubens was an accomplished experimentalist and was working on a problem called “The Ultraviolet Catastrophe” for several years, although it wasn’t called that until 1911. This was a case where experimental data was not lining up with theoretical, mathematical equations that physicists had devised up to that point. After Rubens left, Planck spent the rest of the evening trying come up with a more accurate, less catastrophic equation to fit Ruben’s experimental data.
In one evening, Planck managed to derive a mathematical equation that was in excellent agreement with Ruben’s experimental data. Before retiring to bed that evening, he jotted down his now famous blackbody radiation formula onto a postcard and mailed it to Rubens. What Planck managed to do was reverse engineer the data Ruben’s had accumulated, and arrive at his results by “lucky guess-work.” At that time, Planck did not have a physical interpretation for his formula, he just knew that the numbers worked.
Planck worked for two months to come up with what is now knows as “Planck’s Law,” which describes energy as manifesting in the form of discrete levels, now called “quanta,” instead of the linear manifestation that nearly everyone assumed up to that point. He presented his findings to the German Physical Society on December 14, 1900, the day now widely regarded as the birthday of quantum physics and also a demarcation point between the eras of “classical physics” and “quantum physics.”
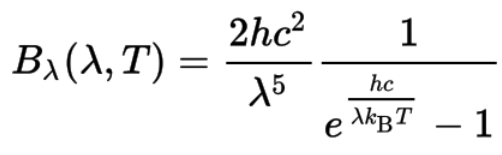
The Ultraviolet Catastrophe
(Rayleigh-Jeans Catastrophe)
Planck’s law solved a problem Physicists were grappling with, now known as The Ultraviolet Catastrophe or Rayleigh-Jeans Catastrophe. This has to do with radiation from so-called black-bodies, theoretical objects that absorb all radiation and reflect none. Think of a fire poker iron, you heat it up and it starts to turn red, heat it up some more and it turns white-hot. This is the concept here. When a blackbody is heated it will give of light, the frequency of which is related to the amount of heat applied to the body.
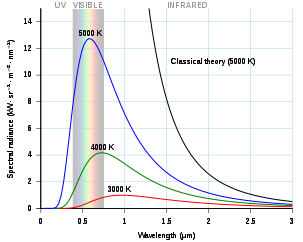
The spectra of light emitted is usually drawn on a graph called a blackbody curve where the intensity of light is the y-axis and wavelength (frequency) of the light emitted on the x-axis. The lines on the graph represent the various range of temperatures.
Classical physicists were working hard on a theoretical description of the blackbody curve. They assumed charged particles (electrons) were being “excited” when heated causing the emission of radiation. They weren’t far off from the truth, as we now know that electrons dropping from higher energy levels to lower energy levels release photons of light energy, although this is a highly simplified way of understanding it.
By 1900 British physicist Lord Rayleigh and Sir James Jeans had devised an equation that accurately aligned with the experimental data for frequencies lower than 10^5 GHZ. Above that, their curve starts to fly off the page into infinity, which was not was observed in the lab.
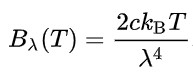
- Bν(v,T) is the spectral radiance (the power per unit solid angle and per unit of area normal to the propagation) density of frequency ν radiation per unit frequency at thermal equilibrium at temperature T
- c is the speed of light in a vacuum
- kB is the Boltzmann constant (1.38064852 × 10-23 m2 kg s-2 K-1)
- ν is the frequency of the electromagnetic radiation
- T is the absolute temperature of the body
The Royal Institute
Max Planck was able to fix this equation by assuming that the energy levels of the oscillating charged particles (electrons) could NOT assume any level, but were bound to certain fixed energy levels. He imagined the energy levels being spaced evenly like rungs on a ladder. The classical view of physics assumed any energy level would be permitted. Thus, a revolution in physics had begun which changed everything.
Quantum Weirdness
Over the next several decades Quantum Theory progressed into a strange world hardly recognizable to classical physicists. Some of the ideas uncovered included the following:
Wave/Particle Duality
Many people probably assume that Albert Einstein won the Nobel Prize in Physics for his now famous Theory of Relativity. However, it was actually for his work on the photoelectric effect which describes light as acting sometimes like a particle (quanta) and sometimes like a wave. Physicist Luis De Brolies picked up on this idea and produced the concept of “matter waves,” the idea that particles of matter exhibit behavior as waves as well as particles.
The Heisenberg Uncertainty Principle
Werner Heisenberg (not the Breaking Bad guy) devised the Heisenberg Uncertainty Principle which expresses the fact that the product of uncertainties in certain pairs of observables (like position and momentum) has a minimum value approximately equal to Planck’s constant. This basically means that you can’t measure things at the atomic level without affecting the measurement in some way, therefore there is always a certain amount of uncertainty involved causing things at the atomic level to be probabilistic in nature. This idea never sat well with Einstein, and he spent a good portion of his career trying to disprove the concept. You see this probabilistic nature of matter at the atomic level is not just caused by imprecise measurements, it’s a fundamental law of life at the microscopic level.
Particles Don’t Exist Until they are Observed
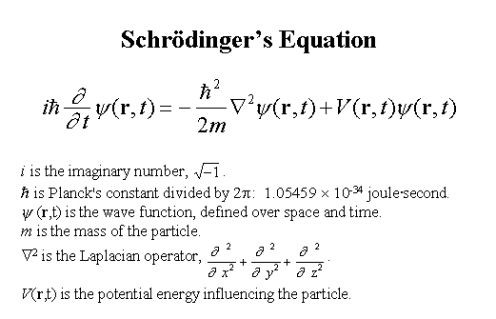
This big ugly mess of math is called Schrodinger’s equation:
University of Graz
It describes matter in terms of wave functions, but once a particle is observed, the wave function collapses into a particle.
Quantum Entanglement
Too complex to go into here, but the quirky thing about this one is that two particles can apparently “communicate” with each other at great distances with speeds greater than the speed of light, which according to Einstein’s theory of relativity is supposed to be impossible.
Many other theories and equations have arisen since the dawn of the quantum age of physics, too numerous to account for here.

